Research Article
Abstract
Respiratory distress continues to be a major cause of neonatal morbidity. Current neonatal practice recommends the use of nasal continuous positive airway pressure (nCPAP) in the immediate resuscitation and continued support of neonates of all gestations with clinical manifestations of respiratory distress. Despite the many short- and long-term benefits of nCPAP, many neonatal care units have met resistance in its routine use. Although there have been numerous recent publications investigating the use and outcomes of various modes of nCPAP delivery, surfactant administration, mechanical ventilation, and other forms of noninvasive respiratory support (high-flow nasal cannula, nasal intermittent positive pressure ventilation), there has been a relative lack of publications addressing the practical bedside care of infants managed on nCPAP. Effective use of nCPAP requires a coordinated interprofessional team approach, ongoing assessment of the neonate, troubleshooting the nCPAP circuit, and parent education.
Respiratory distress at birth is a significant complication that occurs in newborns of all gestations. The cost of respiratory distress in the neonate is high. Conditions associated with respiratory distress include respiratory distress syndrome (RDS), transient tachypnea of the newborn (TTN), bronchopulmonary dysplasia (BPD), air leaks, pulmonary hypoplasia, meconium aspiration syndrome, and persistent pulmonary hypertension of the newborn (PPHN). These diseases account for nearly one-fourth of all NICU deaths. 1 The incidence of RDS increases with decreasing gestational age, affecting 93 percent of neonates born at 28 weeks’ gestation and earlier. 2 However, RDS still occurs in a significant number of late-preterm and term infants. Respiratory distress syndrome has been reported to occur in 10.5 percent of infants born at 34 weeks, 6 percent at 35 weeks, 2.8 percent at 36 weeks, 1 percent at 37 weeks, and 0.3 percent at 38 weeks and greater. 3 Late-preterm and term infants are also prone to other respiratory disorders. The two most common pathologies of respiratory distress in these infants are PPHN and TTN. 4,5
Nasal continuous positive airway pressure (nCPAP) has been used and studied for decades in the care of neonates 6 and is the preferred respiratory support even for premature infants. 7 Nasal continuous positive airway pressure is the standard of care for immediate resuscitation and continued support in neonates of all gestations who breathe spontaneously, but have clinical manifestations of respiratory distress. The use of nCPAP is associated with lower mortality and a lower incidence of respiratory morbidities than intubation with or without surfactant. 8–10 The incidence of BPD, pulmonary air leaks such as pneumothorax, ventilator-associated pneumonia, and neurocognitive disorders is lower with the use of nCPAP than with mechanical ventilation. 11–15
Process failures are common in the use of nCPAP because maintaining nCPAP is a complex and arduous process requiring an astute and motivated interdisciplinary team. The failure rate of nCPAP in premature infants is significant, reported to be as high as 50 percent in infants less than 29 weeks’ gestation, 8–10 and 29 percent at 29–32 weeks’ gestation. 16 Furthermore, neonates who experience nCPAP failure have longer hospitalizations that are complicated by comorbidities such as pneumothorax, BPD, and retinopathy of prematurity. They also have a heightened risk of death. 16 Therefore, despite the many short- and long-term benefits of nCPAP, some neonatal care providers are resistant to using this therapy.
Although there have been a number of recent publications investigating the use and outcomes of various modes of mechanical ventilation, surfactant administration, nCPAP delivery, and other forms of noninvasive respiratory support (high-flow nasal cannula, nasal intermittent positive pressure ventilation), there has been a relative paucity of publications addressing the practical bedside care of infants managed on nCPAP. To prepare this guide, we conducted a comprehensive review of the literature and current evidence to develop a background overview of pathophysiologic mechanisms and practical application of nCPAP at the bedside.
Why Use nCPAP? The Background Pathophysiology
Respiratory distress at birth is typically caused by an impaired transition from fetal to extrauterine life. Clinical signs of respiratory distress include tachypnea (respiratory rate >60 breaths per minute), nasal flaring, intercostal or substernal retractions, accessory muscle use, grunting, and cyanosis. The etiology for neonatal respiratory difficulties most commonly is RDS, TTN, or PPHN.
Respiratory Distress Syndrome
Respiratory distress syndrome is primarily caused by a deficiency of surfactant. Surfactant decreases alveolar surface tension, decreasing the pressure needed to inflate and keep alveoli open. The quantity and quality of surfactant is lower with decreasing gestational age. Portions of surfactant can be synthesized during the saccular phase of lung development, between 27 and 36 weeks’ gestation, but most infants born before 34 weeks’ gestation do not have adequate surfactant to support extrauterine life without respiratory support because mature levels of pulmonary surfactant are not usually present until after 35 weeks’ gestation. 17–19 In addition to prematurity, surfactant deficiency can be associated with the following 20 :
Maternal diabetes
Cesarean section without labor
Fetal asphyxia
Maternal hypertension
Family history
Male sex
Surfactant deficiency results in increased work of breathing as the neonate tries to generate enough pressure to open alveoli and inflate the lungs. This increase in work of breathing is accentuated by the increase in chest wall compliance seen in both preterm and term infants. 21,22 In addition to having a highly compliant chest wall, premature infants are prone to central and obstructive apnea, 23 and mature alveoli are not present until 36 weeks’ gestation. 20 These factors all contribute to diffuse atelectasis, which results in decreased lung compliance and a low functional residual capacity (FRC), ultimately causing a mismatch of ventilation and perfusion. Blood bypasses collapsed, unoxygenated alveoli, causing a ventilation-to-perfusion mismatch resulting in hypoxemia (Figure 1). This hypoxemia can then contribute to an increase in pulmonary vascular resistance, resulting in increased right-to-left shunting of deoxygenated blood through the foramen ovale and ductus arteriosus, further worsening arterial hypoxemia and ultimately resulting in respiratory failure.
Abbreviation: TTN = transient tachypnea of the newborn.

Respiratory distress syndrome presents with increased work of breathing, desaturation, and cyanosis immediately at or shortly after birth. The signs of RDS tend to progress over the first 24–72 hours of life and then begin to stabilize and improve as the newborn’s body starts to produce surfactant in response to birth. Exogenous surfactant can be given via endotracheal intubation or minimally invasive methods, but early CPAP has been shown to decrease the need for intubation and surfactant administration. 24,25 Antenatal glucocorticoids promote the maturation of alveoli and surfactant production and have significantly decreased the incidence and severity of RDS in very premature and late-preterm infants. 26,27 Nasal continuous positive airway pressure maintains and can even increase FRC by exceeding the closing capacity of the lungs. Therefore, nCPAP can improve ventilation and ventilation–perfusion mismatch, prevent progressive arterial hypoxemia, decrease respiratory muscle fatigue, and ultimately reduce the failure seen in the setting of RDS. 23
Transient Tachypnea of the Newborn
Transient tachypnea of the newborn is a result of the failure of adequate lung fluid clearance at birth. It is an increase in sodium and lung fluid reabsorption during labor and delivery rather than the mechanical squeezing of the fetus passing through the birth canal that clears the excess fluid in the fetus’s lungs. 28 Fetal lung fluid is produced by the lung itself, and amniotic fluid rarely enters the developing lung except during fetal distress. 23 The rate of secretion of lung fluid starts to decrease two to three days before birth, with nearly two-thirds of the total clearance of fluid occurring during labor. 23,29 The onset of labor stimulates the pulmonary epithelium to change from a chloride-secreting membrane to a sodium-absorbing membrane, resulting in absorption of fetal lung fluid. 28 This process is developmentally regulated, with premature infants being less able to clear fetal lung fluid. The accumulated fluid leads to compression of compliant airways, gas trapping, hypoxemia from ventilation–perfusion mismatch, and hypercarbia from interference with alveolar ventilation. 23
Transient tachypnea of the newborn typically appears as tachypnea within two hours of birth and resolves by 72 hours after birth, with respiratory improvement coinciding with the natural diuresis that tends to occur around this time period. Transient tachypnea of the newborn occurs more frequently in infants after an elective cesarean birth, because these infants are born without the benefit of labor. Changes in stress hormones and the rapid clearance of fetal lung fluid, largely through sodium reabsorption, are inadequate or not present in preterm gestations or the absence of labor, which places the neonate at risk for respiratory distress. 30 Transient tachypnea of the newborn also occurs more frequently in twin pregnancies, preterm birth, maternal diabetes, and maternal asthma. Male sex and higher birth weights are associated with an increased incidence of TTN. 31,32 Nasal continuous positive airway pressure improves oxygenation in infants with TTN by stenting open airways, increasing alveolar pressure to expand alveoli, and promoting clearance of lung fluid, which decreases ventilation–perfusion mismatch (see Figure 1).
Persistent Pulmonary Hypertension of the Newborn
Persistent pulmonary hypertension of the newborn is a result of the neonate’s inability to decrease pulmonary vascular resistance after birth. During intrauterine life, pulmonary vascular resistance is high because little blood flow needs to go to the lungs in the absence of breathing air. Therefore, the fetus lives in a relatively hypoxic environment. With the first breaths at birth, oxygen in the air is introduced through the lungs to the pulmonary vasculature, resulting in vasodilation and a decrease in pulmonary artery pressures. Blood flow to the lungs is then increased.
With PPHN, pulmonary pressures fail to decrease in the newborn, leading to right-to-left shunting of deoxygenated blood across the foramen ovale and ductus arteriosus, resulting in cyanosis. This hypoxemia leads to respiratory distress as the infant attempts to compensate for the decreased oxygen levels and attempts to open alveoli to increase oxygen delivery to the pulmonary vasculature. Neonates with PPHN typically present with tachypnea and cyanosis. Because of right-to-left shunting, differential pre- and postductal saturations are common. Arterial blood gases show severe hypoxemia and oxygen desaturation, often with normal carbon dioxide tensions. 33 Nasal continuous positive airway pressure is used in the treatment of PPHN to provide both lung inflation and oxygenation, which results in a decrease in pulmonary vascular resistance.
How Does CPAP Work?
Nasal continuous positive airway pressure is a noninvasive method of maintaining lung inflation by applying a constant pressure to keep the alveoli open during inhalation and exhalation. Continuous distending pressure (nCPAP; heated humidified high-flow nasal cannula [HHHFNC]) improves diaphragm function, reduces upper and lower airway resistance, increases pulmonary compliance, improves tidal volumes, and decreases alveolar edema. 34 The clinical goals of nCPAP are to maintain the FRC of the neonate’s lungs and support gas exchange, thereby decreasing the work of breathing, improving oxygenation and ventilation, and potentially decreasing both obstructive and central apnea. Nasal continuous positive airway pressure works well in neonates as they are obligate nasal breathers and pressure is maintained in the lungs because of the anatomic seal that forms between the neonate’s tongue and soft palate. Three types of nCPAP are commonly used in the clinical setting. 35
Constant Flow/Ventilator-Derived nCPAP
Constant flow/ventilator-derived nCPAP is one of the oldest forms of CPAP and uses a ventilator to provide a set flow and positive end expiratory pressure (PEEP). It has been in use the longest; however, it can be associated with an increase in work of breathing when compared with variable flow delivery systems. Because the flow does not vary with the infant’s respiratory cycle with this delivery system, the infant must work harder to exhale against the positive pressure. 36 Other forms of delivering nCPAP have gained favor over the years. Bubble nCPAP, in particular, has been shown to have several advantages over ventilator-derived constant flow nCPAP. 37–40
Variable Flow nCPAP/Infant Flow
With variable flow or fluidic flip nCPAP, a device (driver) incorporates an oxygen blender, flowmeter, and pressure monitor to deliver the necessary amount of nCPAP. Variable flow systems allow for increased pressure support during inhalation and decreased resistance during exhalation. This can result in increased lung volumes, improved lung compliance, decreased work of breathing, and improved lung recruitment when compared with constant flow systems. 41 This system has many advantages including pressure display, alarms, and an internal pressure relief valve that allows for a more constant pressure delivery while minimizing excessive pressure delivery. This device is well tolerated and effective. 42,43
Bubble nCPAP
Bubble nCPAP uses positive pressure by immersing the expiratory tubing in a water column to deliver pressure. With bubble nCPAP, there is not a machine that delivers nCPAP; rather, the depth in centimeters of the expiratory tubing delivers the desired PEEP. A flowmeter attached to a blender and cylinder with water (acetic acid can be used to deter bacterial growth) is the mechanism for positive pressure generation (Figure 2). There are no alarms for this setup; therefore, vigilance at the bedside is required to assure proper connections, positioning, pressure delivery, and respiratory support. Bubble nCPAP delivers mechanical oscillatory vibrations that simulate ventilation in a similar way to high-frequency ventilation. 44 With bubble nCPAP, nonrecruited areas of the lung may open and air exchange may be improved because of the variable frequency and amplitude of the pressure oscillations. 23 These pressure oscillations provide enhanced ventilation, and Lee and colleagues 38 found that bulk tidal volumes, respiratory rates, and minute ventilation were lower in premature infants supported with bubble nCPAP when compared with ventilator nCPAP. In addition, there was no statistically significant difference in transcutaneous carbon dioxide pressure or oxygen saturations between the two groups. 38 Infants using bubble nCPAP have also been shown to have fewer complications, a decreased need for intubation, less likelihood of respiratory failure, and shorter hospital stays, all at a lower cost than ventilator-derived nCPAP. 37,39,40 Although initially employed in premature infants, bubble nCPAP is now being used for infants of all gestations.

Heated Humidified High-Flow Nasal Cannula
Heated humidified high-flow nasal cannula is an alternative method to deliver continuous distending pressure without nCPAP. Heated humidified high-flow nasal cannula was developed as an alternative to nCPAP systems because of perceived improved patient tolerance and ease of use. 12 Heated humidified high-flow nasal cannula uses a flowmeter and heated, humidified oxygen delivered at high rates via nasal cannula prongs. The flow rate needed is determined by the physician or advanced practice nurse based on the clinical needs of the patient. Our group uses flow rates ranging from 2 to 5 L/minute. A recent Cochrane review found that in comparison with other forms of noninvasive respiratory support, high-flow nasal cannula has similar rates of efficacy for preventing extubation failure, death, and chronic lung disease. The review also found that nasal trauma and potential pneumothorax are also decreased with the use of high-flow nasal cannula. 45 The primary concern with HHHFNC centers on the highly variable pressure delivery because it is the flow rate and not the exact pressure that is delivered in this system; the high-flow air generates a continuous positive airway pressure, but the amount of pressure generated is not measured or regulated. Iyer and Mhanna 46 showed a significant linear association between flow rate and distending pressures in premature infants with a mean gestational age of 26.6 weeks, but commented that the pressures generated by a specific flow rate were variable. Heated humidified high-flow nasal cannula is predominantly used when weaning the neonate from nCPAP; however, Yoder and associates found that HHHFNC had the same clinical efficacy and safety when compared with nCPAP in neonates ≥ 28 weeks’ gestation. 47 Several studies have shown HHHFNC is well tolerated by premature infants, may improve growth, and did not increase the risk of death, BPD, or other outcomes. 12,47 However, the effective use of HHHFNC has been debated because a large meta-analysis of clinical trials did not show HHHFNC as equivalent or superior to nCPAP, and concluded that the safety and efficacy of HHHFNC in extremely preterm and mildly preterm infants still needs to be established. 45 Therefore, many institutions choose to only use HHHFNC in infants >32 weeks corrected gestational age who are in a convalescent phase.
Key Points in the Use of nCPAP
Nasal continuous positive airway pressure can be delivered via nasal prongs or mask used with a hat or headgear. Several different devices are available on the market for nCPAP delivery. A key point in the effective use of nCPAP is properly fitting equipment. It is paramount that the hat and prongs/mask remain in proper position to maintain CPAP flow and prevent pressure injuries. In addition, proper positioning of the neonate to maximize airway patency is necessary. It is important to know that with bubble nCPAP, bubbling is present when there is an open functional circuit (the goal); however, it is also present when the circuit is obstructed. Therefore, it is important to reassess the neonate and nCPAP system on a regular basis.
Excessive movement, especially in late-preterm and term infants, can dislodge the nCPAP system. Comfort measures such as swaddling, decreasing environmental stimulation, skin-to-skin contact, use of a pacifier, and presence of the caregiver can calm the neonate to allow maximum delivery of pressure to prevent further complications. Effective nCPAP requires a multidisciplinary team approach that includes the family. A successful nCPAP program involves:
Physicians and advanced practice nurses to apply evidence-based medicine to support nCPAP use
Respiratory therapists to setup and maintain the nCPAP (starting in the delivery room)
Nurses providing bedside care and continually reassessing the infant
Occupational therapy helping with developmental positioning and appropriate stimulation
Pharmacy and nutrition teams to assure adequate nutrient delivery and monitor growth
The family to provide skin-to-skin care (which can be done on any form of respiratory support), offer developmental stimulation, and be the advocate for their child
It is vital for the entire team to understand the importance of nCPAP and be willing and able to support and help each other make nCPAP successful.
Assessing the Neonate on nCPAP
Assessment is paramount in ensuring the neonate is receiving adequate ventilation and that skin integrity is maintained on nCPAP. Frequent assessments are essential, and the CPAP device should be removed every four to six hours to adequately evaluate the skin for signs of compromise. 18
Skin/Tissue Integrity
There are many potential pressure points on the head and face when using CPAP. The nares, columella, and nasal septum are particularly at risk for injury because of the positioning of prongs or a mask. Properly fitted prongs will avoid pressure on these high-risk areas as well as prevent excess movement and rubbing. A properly sized and positioned mask is needed to prevent slipping and pressure injury. With a mask, pressure injury can occur to the nasal bridge and/or to the nares and columella. In addition, the mask can slide upward and obstruct the nares. Depending on the hat and strap apparatus used, pressure may also occur on the cheeks, scalp, chin, and ears. Therefore, it is important to remove the hat and chin strap completely to do a full assessment.
Effectiveness of nCPAP Delivery and Support of Ventilation
Regular assessments of the neonate’s color, work of breathing, and oxygen saturation are mandatory, as is evaluating respiratory rate, symmetry of chest movement, and breath sounds. Retractions, grunting, and increased work of breathing need immediate attention and may necessitate an increase in ventilatory support. When auscultating breath sounds, the clinician should be able to hear the CPAP bubbling or roar throughout the entire chest when CPAP is being delivered appropriately. Unequal chest movement or a sudden increase in respiratory rate and/or work of breathing may indicate a pneumothorax or other pulmonary complication.
Proper nCPAP Positioning and Equipment Application
The hat should be at the brow line (Figure 3), cover the ears (ensure the ears are not folded over), and reach the base of the neck. The hat should fit snugly so it will hold the CPAP apparatus in place but not be so tight that it causes blanching of the skin or pulls up on the CPAP mask/prongs, lifting the nose toward the forehead. The lateral straps should have equal tension on them and should not cut into or cause an indentation in the facial cheeks. The nCPAP circuit should be secured so that any condensation drains toward the nCPAP unit and the tubing does not slip out of the incubator, causing tension against the headgear and the baby’s face.
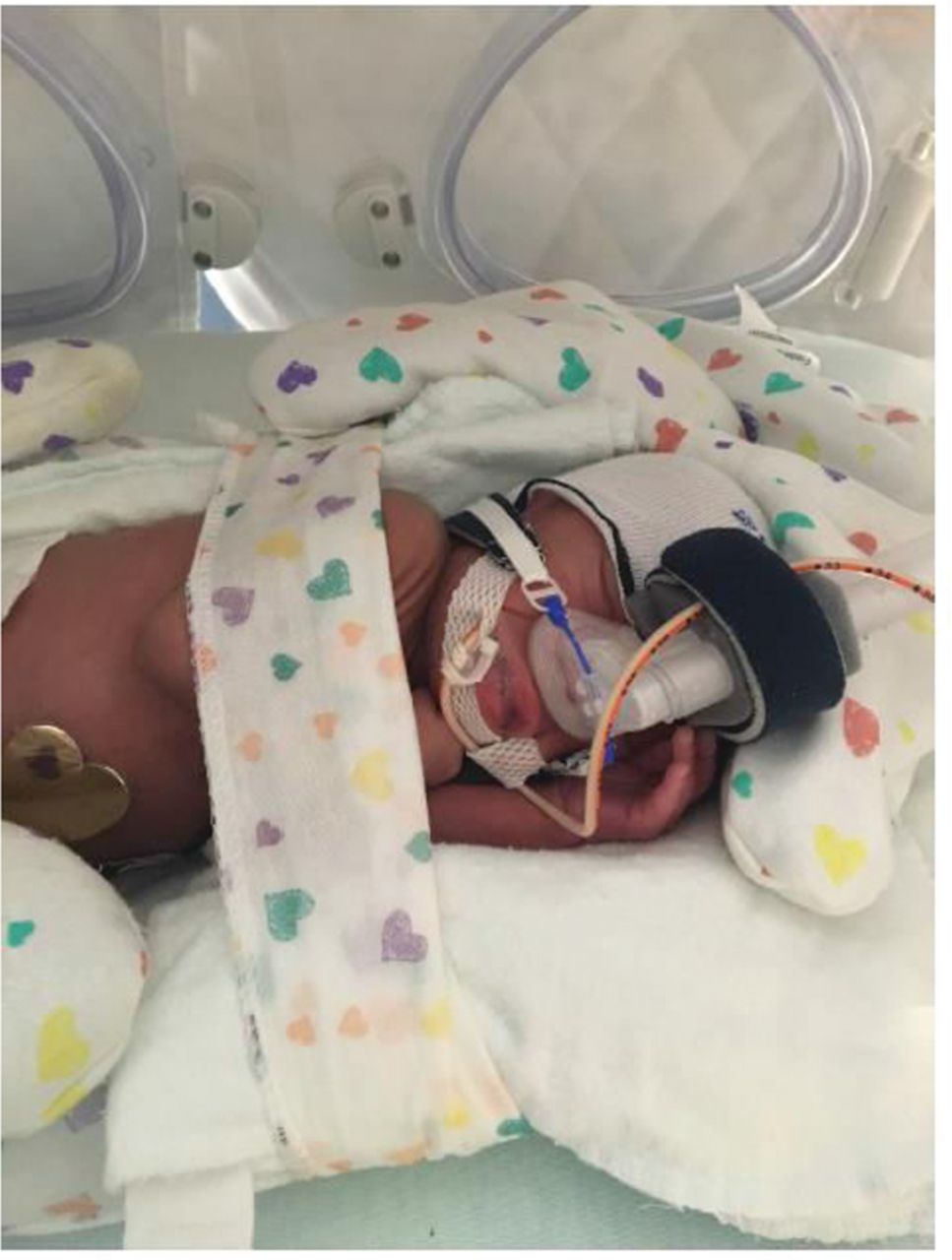
Positioning the neonate so the mouth remains closed is important because an open mouth may decrease the pressure delivered and increase the possibility of respiratory distress. Placing the neonate in the prone position with his/her hand tucked underneath the chin may help keep the mouth closed. Using a roll under the neck or chest may help keep the airway patent by facilitating flow past the nasopharyngeal level. A chin strap is also helpful to use when the infant’s mouth habitually remains open. When using a chin strap, it is important that it is positioned underneath the bony portion of the chin and not over the fleshy portion of the hypopharynx region because this may cause airway obstruction and place the infant at risk for aspiration.
Manufacturers supply a template for proper selection of the size of nasal prongs and masks. The template and choices for the different sizes differ based on the manufacturer. Nasal prongs should fill the entire nares but not cause blanching. There should be a small (several millimeters) cushion of air between the nasal septum and the base (crossbar) of the prongs. The nares should be kept clear of secretions but deep suctioning should be minimized to avoid trauma and resultant edema of the nasal mucosa. The mask should cover the entire nose, yet be small enough to sit flush with the face and not slide. The mask should not fit too tightly; it should not cause indentations and pressure on the nasal bridge or ride up and occlude the nares. 40 Alternating the use of mask and prongs can help minimize pressure injuries.
Abdominal Distention
Early in the use of nCPAP for neonates, there was concern for increased risks of gastrointestinal perforation and necrotizing enterocolitis, but this has not been seen in recent studies. 34,48 The use of nCPAP, however, has been associated with abdominal distention in neonates, especially those born prematurely. This is a result of air being pushed into the stomach and is complicated by the gastrointestinal dysmotility commonly seen in sick and premature neonates. It is important to continually assess the abdomen for distention, discoloration, and tenderness. An appropriately sized orogastric tube (at least 8 Fr, if permitted by the infant’s size) should always be in place when nCPAP is in use. This will allow for the escape of gastric air that is under pressure. If this gastric air reaches the intestines, it causes gaseous distention of the bowel that will only be removed when the infant passes the air rectally. Feeding tolerance should also be monitored closely and the orogastric tube should be allowed to vent between feedings.
Assessing and Troubleshooting the nCPAP Circuit
Whenever there is concern about the baby’s well-being or the integrity of the nCPAP delivery system, it is critical to assess the neonate first, then the respiratory support system. The nCPAP circuit and equipment should be evaluated any time that the neonate’s respiratory status is not improving as expected or if the infant shows signs of increasing respiratory distress, has an increasing oxygen need, is clinically deteriorating, and/or shows signs of increasing agitation (Table 1).
| Circuit setup and configuration |
|
| Maintaining ordered pressure |
|
| Water in the circuit |
|
[i] Abbreviations: CPAP = continuous positive airway pressure; PEEP = positive end expiratory pressure.
Danger Signs
Critically ill neonates can change their clinical course at any moment. Whether occurring at the time of birth, during treatment, or during the weaning period, it is critical to identify impending danger signs, necessitating action from the neonatal team. Difficulties with maintaining nCPAP include complicated application techniques, maintenance of the delivery circuit, positional problems, nasal damage, and neonatal agitation. 22 Danger signs and targeted assessments and interventions are located in Table 2.
| Danger Signs | Suggested Action |
|---|---|
| Clinical change: increased work of breathing, increased alarms (apnea, bradycardia, desaturations), increase in oxygen need |
|
| |
| redness, blanching, erosion, excoriation |
|
| unable to maintain pressure, flow, oxygen delivery, bubbling |
|
[i] Abbreviations: CPAP = continuous positive airway pressure; OG = orogastric; PEEP = positive end expiratory pressure.
Helping Parents and Caregivers Cope with a Neonate on nCPAP
Bedside management of a neonate on CPAP is very time-consuming and care intensive. It is important to review the importance of successful maintenance of nCPAP with caregivers and families. As new alveolar formation continues throughout the first two to three years of postnatal life, 17 adequately supporting a neonate with noninvasive ventilation can avoid the short- and long-term complications of intubation and mechanical ventilation and allow for normal lung formation and function later in life. The benefits of nCPAP are summarized in Table 3 and outweigh the risks of tissue injury and treatment failure, which can be minimized thorough interdisciplinary care.
Development/
g
rowth
|
Respiratory
|
Financial
|
[i] Abbreviations: BPD= bronchopulmonary dysplasia; CPAP= continuous positive airway pressure.
Conclusion
Nasal CPAP is used widely in the NICU setting for the management of neonates with a variety of respiratory ailments and has been shown to have multiple benefits alone and in comparison with mechanical ventilation. The successful maintenance of nCPAP depends on meticulous bedside care that requires coordination between physicians, nurses, advanced practice nurses, respiratory therapists, occupational therapists, dietitians, pharmacists, the skin team, and the family. Because nCPAP is used for infants of all gestational ages, a developmental approach is necessary and an understanding of the underlying pathophysiology is helpful in targeting assessments and troubleshooting approaches. Involvement and feedback from all members of the neonatal care team are essential to provide proper care of these infants and optimize outcomes.
Continuing Nursing Education (CNE) Credit
Attention Readers: The test questions are provided in this issue, but the posttest and evaluation must be completed online. Details to complete the course are provided online at academyonline.org/CNE. A total of 1.5 contact hour(s) may be earned as CNE credit for reading this article and completing the online posttest and evaluation. To be successful the learner must obtain a grade of at least 80% on the test. Test expires three (3) years from publication date. Disclosure: The authors/planning committee have no relevant financial interest or affiliations with any commercial interests related to the subjects discussed within this article. No commercial support or sponsorship was provided for this educational activity. ANN/ANCC does not endorse any commercial products discussed/displayed in conjunction with this educational activity.
The Academy of Neonatal Nursing is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation.
Provider, Academy of Neonatal Nursing, approved by the California Board of Registered Nursing, Provider #CEP 6261; and Florida Board of Nursing, Provider #FBN 3218, content code 2505.
The purpose of this article is to share the evidence supporting nasal CPAP for neonates with respiratory distress and the nursing care of these infants.
References
- Jacob J , Kamitsuka M , Clark RH , Kelleher AS , Spitzer AR . Etiologies of NICU deaths. Pediatrics. 2015;135(1):e59–e65. 10.1542/peds.2014-2967
- . Stoll BJ , Hansen NI , Bell EF , et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456. 10.1542/peds.2009-2959
- Hibbard JU , Wilkins I , Sun L , et al. Consortium on Safe Labor. Respiratory morbidity in late preterm births. JAMA. 2010;304(4):419–425. 10.1001/jama.2010.1015
- Hagen E , Chu A , Lew C . Transient tachypnea of the newborn. Neoreviews. 2017;18(3):8:e141–e148. 10.1542/neo.18-3-e141
- Sharma V , Berkelhamer S , Lakshminrusimha S . Persistent pulmonary hypertension of the newborn. Matern Health Neonatol Perinatol. 2015;1:14. 10.1186/s40748-015-0015-4
- De Paoli AG , Davis PG , Faber B , Morley CJ . Devices and pressure sources for the administration of nCPAP in preterm neonates. Cochrane Database Syst Rev. 2002;4:CD002977.
- Committee on Fetus and Newborn, American Academy of Pediatrics. Respiratory support in preterm infants at birth. Pediatrics. 2014;133(1):171–174. 10.1542/peds.2013-3442
- Dunn MS , Kaempf J , de Klerk A , et al. Vermont Oxford Network DRM Study Group. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics. 2011;128(5):e1069–e1076. 10.1542/peds.2010-3848
- Morley CJ , Davis PG , Doyle LW , Brion LP , Hascoet JM , Carlin JB ; COIN Trial Investigators. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med. 2008;358(7):700–708. 10.1056/NEJMoa072788
- Finer NN , Carlo WA , Walsh MC , et al. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–1979. 10.1056/NEJMoa0911783
- Fischer HS , Bührer C . Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics. 2013;132(5):e1351–e1360. 10.1542/peds.2013-1880
- Holleman-Duray D , Kaupie D , Weiss MG . Heated humidified high-flow nasal cannula: use and a neonatal early extubation protocol. J Perinatol. 2007;27(12):776–781. 10.1038/sj.jp.7211825
- Isayama T , Chai-Adisaksopha C , McDonald SD . Noninvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 2015;169(8):731–739. 10.1001/jamapediatrics.2015.0510
- Schmölzer GM , Kumar M , Pichler G , Aziz K , O'Reilly M , Cheung PY . Non-invasive versus invasive respiratory support in preterm infants at birth: systematic review and meta-analysis. BMJ. 2013;347:f5980. 10.1136/bmj.f5980
- Subramaniam P , JJ H , Davis PG . Prophylactic nasal continuous positive airway pressure for preventing morbidity and mortality in very preterm infants. Cochrane Database Syst Rev. 2006;6:CD001243.
- Dargaville PA , Gerber A , Johansson S , et al. Australian and New Zealand Neonatal Network. Incidence and outcome of CPAP failure in preterm infants. Pediatrics. 2016;138(1):e20153985. 10.1542/peds.2015-3985
- Guttentag S , Ballard PL . Lung development: embryology, growth, maturation, and developmental biology. In: Taeusch HW , Ballard RA , Gleason CA , eds. Avery’s Diseases of the Newborn. 8th ed. Philadelphia, PA: Elsevier Saunders; 2005:601–615.
- Casey JL , Newberry D , Jnah A . Early bubble continuous positive airway pressure: investigating interprofessional best practices for the NICU team. Neonatal Netw. 2016;35(3):125–134. 10.1891/0730-0832.35.3.125
- Pickerd N , Kotecha S . Pathophysiology of respiratory distress syndrome. Paediatr Child Health. 2009;19(4):153–157. 10.1016/j.paed.2008.12.010
- Taeusch HW , Ramierez-Schrempp D , Laing IA . Surfactant treatment of respiratory disorders. In: Taeusch HW , Ballard RA , Gleason CA , eds. Avery’s Diseases of the Newborn. 8th ed. Philadelphia, PA: Elsevier Saunders; 2005:670–685.
- Loper DL . Physiologic principles of the respiratory system. In: Askin DF , ed. Acute Respiratory Care of the Neonate. 2nd ed. Petaluma, CA: NICU Ink; 1997:1–30.
- Nichols DG , Rogers MC . Developmental physiology of the respiratory system. In: Rogers MC , ed. Textbook of Pediatric Intensive Care. Baltimore, MD: Williams & Wilkins; 1987:83–111.
- Kribs A . Minimally invasive surfactant therapy and noninvasive respiratory support. Clin Perinatol. 2016;43(4):755–771. 10.1016/j.clp.2016.07.010
- Rojas-Reyes MX , Morley CJ , Soll R . Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2012;14(3):Art. No. CD000510. 10.1002/14651858.CD000510.pub2
- Saccone G , Berghella V . Antenatal corticosteroids for maturity of term or near term fetuses: systematic review and meta-analysis of randomized controlled trials. BMJ. 2016;355:i5044. 10.1136/bmj.i5044
- Roberts D , Dalziel S . Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;19(3):CD004454. 10.1002/14651858.CD004454.pub2
- Yurdakök M . Transient tachypnea of the newborn: what is new? J Matern Fetal Neonatal Med. 2010;23(Suppl 3):24–26. 10.3109/14767058.2010.507971
- Bland RD . Dynamics of pulmonary water before and after birth. Acta Paediatr Scand Suppl. 1983;305:12–20. 10.1111/j.1651-2227.1983.tb09853.x
- Jain L . Stress at birth and its inextricable link to the neonatal transition. Obstet Gynecol. 2016;128(4):685–687. 10.1097/AOG.0000000000001657
- Edwards MO , Kotecha SJ , Kotecha S . Respiratory distress of the term newborn infant. Paediatr Respir Rev. 2013;14(1):29–37. 10.1016/j.prrv.2012.02.002
- Sweet DG , Carnielli V , Greisen G , et al. European Association of Perinatal Medicine. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants--2013 update. Neonatology. 2013;103(4):353–368. 10.1159/000349928
- Ballard RA , Hansen TN , Corbet A . Respiratory failure in the term infant. In: Taeusch HW , Ballard RA , Gleason CA , eds. Avery’s Diseases of the Newborn. 8th ed. Philadelphia, PA: Elsevier Saunders; 2005:705–722.
- Esmaeilnia T , Nayeri F , Taheritafti R , Shariat M , Moghimpour-Bijani F . Comparison of complications and efficacy of NIPPV and nasal CPAP in preterm infants with RDS. Iran J Pediatr. 2016;26(2):e2352. 10.5812/ijp.2352
- Diblasi RM . Nasal continuous positive airway pressure (CPAP) for the respiratory care of the newborn infant. Respir Care. 2009;54(9):1209–1235.
- Pandit PB , Courtney SE , Pyon KH , Saslow JG , Habib RH . Work of breathing during constant- and variable-flow nasal continuous positive airway pressure in preterm neonates. Pediatrics. 2001;108(3):682–685. 10.1542/peds.108.3.682
- Bahman-Bijari B , Malekiyan A , Niknafs P , Baneshi MR . Bubble-CPAP vs. ventilatory-CPAP in preterm infants with respiratory distress. Iran J Pediatr. 2011;21(2):151–158.
- Lee KS , Dunn MS , Fenwick M , Shennan AT . A comparison of underwater bubble continuous positive airway pressure with ventilator-derived continuous positive airway pressure in premature neonates ready for extubation. Biol Neonate. 1998;73(2):69–75. 10.1159/000013962
- Martin S , Duke T , Davis P . Efficacy and safety of bubble CPAP in neonatal care in low and middle income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2014;99(6):F495–F504. 10.1136/archdischild-2013-305519
- McCoskey L . Nursing care guidelines for prevention of nasal breakdown in neonates receiving nasal CPAP. Adv Neonatal Care. 2008;8(2):116–124. 10.1097/01.ANC.0000317260.99072.ae
- Pandit PB , Courtney SE , Pyon KH , Saslow JG , Habib RH . Work of breathing during constant- and variable-flow nasal continuous positive airway pressure in preterm neonates. Pediatrics. 2001;108(3):682–685. 10.1542/peds.108.3.682
- Mazmanyan P , Mellor K , Doré CJ , Modi N . A randomised controlled trial of flow driver and bubble continuous positive airway pressure in preterm infants in a resource-limited setting. Arch Dis Child Fetal Neonatal Ed. 2016;101(1):16–20. 10.1136/archdischild-2015-308464
- Gupta S , Sinha SK , Tin W , Donn SM . A randomized controlled trial of post-extubation bubble continuous positive airway pressure versus Infant Flow Driver continuous positive airway pressure in preterm infants with respiratory distress syndrome. J Pediatr. 2009;154(5):645–650. 10.1016/j.jpeds.2008.12.034
- Wung JT . Respiratory management for low-birth-weight infants. Crit Care Med. 1993;21(9 Suppl):S364–365. 10.1097/00003246-199309001-00040
- Wilkinson D , Andersen C , O'Donnell CP , De Paoli AG , Manley BJ . High flow nasal cannula for respiratory support in preterm infants. Cochrane Database Syst Rev. 2016;2:CD006405. 10.1002/14651858.CD006405.pub3
- Iyer NP , Mhanna MJ . Association between high-flow nasal cannula and end-expiratory esophageal pressures in premature infants. Respir Care. 2016;61(3):285–290. 10.4187/respcare.04317
- Yoder BA , Stoddard RA , Li M , et al. Heated, humidified high-flow nasal cannula versus nasal CPAP for respiratory support in neonates. Pediatrics. 2013;131(5):e1482–e1490. 10.1542/peds.2012-2742
- Aly H , Massaro AN , Hammad TA , Narang S , Essers J . Early nasal continuous positive airway pressure and necrotizing enterocolitis in preterm infants. Pediatrics. 2009;124(1):205–210. 10.1542/peds.2008-2588
Figures
Abbreviation: TTN = transient tachypnea of the newborn.
 View in Context
View in Context View in Context
View in ContextTables
| Circuit setup and configuration |
|
| Maintaining ordered pressure |
|
| Water in the circuit |
|
[i] Abbreviations: CPAP = continuous positive airway pressure; PEEP = positive end expiratory pressure.
| Danger Signs | Suggested Action |
|---|---|
| Clinical change: increased work of breathing, increased alarms (apnea, bradycardia, desaturations), increase in oxygen need |
|
| |
| redness, blanching, erosion, excoriation |
|
| unable to maintain pressure, flow, oxygen delivery, bubbling |
|
[i] Abbreviations: CPAP = continuous positive airway pressure; OG = orogastric; PEEP = positive end expiratory pressure.
Development/
g
rowth
|
Respiratory
|
Financial
|
[i] Abbreviations: BPD= bronchopulmonary dysplasia; CPAP= continuous positive airway pressure.
| Period | Abstract | Full | Total | |
|---|---|---|---|---|
| Feb 2025 | 662 | 75 | 6 | 743 |
| Jan 2025 | 820 | 109 | 18 | 947 |
| Dec 2024 | 590 | 101 | 18 | 709 |
| Nov 2024 | 940 | 103 | 14 | 1057 |
| Oct 2024 | 1010 | 81 | 10 | 1101 |
| Sep 2024 | 945 | 89 | 24 | 1058 |
| Aug 2024 | 970 | 79 | 22 | 1071 |
| Jul 2024 | 918 | 91 | 8 | 1017 |
| Jun 2024 | 945 | 100 | 11 | 1056 |
| May 2024 | 1208 | 92 | 15 | 1315 |
| Apr 2024 | 768 | 61 | 8 | 837 |
| Mar 2024 | 318 | 53 | 17 | 388 |
| Feb 2024 | 1073 | 39 | 6 | 1118 |
| Jan 2024 | 189 | 93 | 4 | 286 |
| Dec 2023 | 636 | 45 | 6 | 687 |
| Nov 2023 | 894 | 53 | 10 | 957 |
| Oct 2023 | 856 | 51 | 10 | 917 |
| Sep 2023 | 989 | 49 | 8 | 1046 |
| Aug 2023 | 833 | 34 | 11 | 878 |
| Jul 2023 | 913 | 49 | 11 | 973 |
| Jun 2023 | 1608 | 58 | 7 | 1673 |
| May 2023 | 1439 | 64 | 16 | 1519 |
| Apr 2023 | 1618 | 73 | 20 | 1711 |
| Mar 2023 | 1884 | 72 | 12 | 1968 |
| Feb 2023 | 1346 | 55 | 19 | 1420 |
| Jan 2023 | 2408 | 58 | 23 | 2489 |
| Dec 2022 | 22 | 41 | 16 | 79 |
| Nov 2022 | 296 | 33 | 14 | 343 |
| Oct 2022 | 1198 | 41 | 11 | 1250 |
| Sep 2022 | 1237 | 52 | 23 | 1312 |
| Aug 2022 | 1111 | 34 | 16 | 1161 |
| Jul 2022 | 244 | 38 | 20 | 302 |
| Jun 2022 | 1047 | 30 | 9 | 1086 |
| May 2022 | 295 | 32 | 22 | 349 |
| Apr 2022 | 1525 | 43 | 21 | 1589 |
| Mar 2022 | 2292 | 47 | 23 | 2362 |
| Feb 2022 | 1535 | 47 | 25 | 1607 |
| Jan 2022 | 21 | 31 | 18 | 70 |
| Dec 2021 | 13 | 24 | 7 | 44 |
| Nov 2021 | 5 | 18 | 6 | 29 |
| Oct 2021 | 19 | 43 | 13 | 75 |
| Sep 2021 | 246 | 35 | 20 | 301 |
| Aug 2021 | 1158 | 58 | 12 | 1228 |
| Jul 2021 | 1143 | 31 | 11 | 1185 |
| Jun 2021 | 1296 | 39 | 20 | 1355 |
| May 2021 | 1540 | 60 | 14 | 1614 |
| Apr 2021 | 2003 | 76 | 21 | 2100 |
| Mar 2021 | 2521 | 65 | 26 | 2612 |
| Feb 2021 | 2618 | 72 | 24 | 2714 |
| Jan 2021 | 2631 | 47 | 20 | 2698 |
| Dec 2020 | 812 | 41 | 19 | 872 |
| Nov 2020 | 19 | 64 | 28 | 111 |
| Oct 2020 | 159 | 37 | 19 | 215 |
| Sep 2020 | 1944 | 59 | 23 | 2026 |
| Aug 2020 | 2632 | 44 | 10 | 2686 |
| Jul 2020 | 1440 | 53 | 33 | 1526 |
| Jun 2020 | 259 | 54 | 16 | 329 |
| May 2020 | 1926 | 51 | 22 | 1999 |
| Apr 2020 | 1887 | 64 | 28 | 1979 |
| Mar 2020 | 2392 | 51 | 19 | 2462 |
| Feb 2020 | 2430 | 50 | 25 | 2505 |
| Jan 2020 | 2443 | 61 | 22 | 2526 |
| Dec 2019 | 2051 | 45 | 17 | 2113 |
| Nov 2019 | 2165 | 46 | 25 | 2236 |
| Oct 2019 | 2164 | 56 | 20 | 2240 |
| Sep 2019 | 1904 | 49 | 13 | 1966 |
| Aug 2019 | 774 | 72 | 20 | 866 |
| Jul 2019 | 831 | 35 | 50 | 916 |
| Jun 2019 | 944 | 63 | 36 | 1043 |
| May 2019 | 834 | 68 | 33 | 935 |
| Apr 2019 | 44 | 23 | 18 | 85 |
| Mar 2019 | 86 | 8 | 11 | 105 |
| Feb 2019 | 82 | 7 | 5 | 94 |
| Jan 2019 | 60 | 10 | 9 | 79 |
| Dec 2018 | 52 | 8 | 7 | 67 |
| Nov 2018 | 69 | 8 | 7 | 84 |
| Oct 2018 | 88 | 10 | 14 | 112 |
| Sep 2018 | 49 | 1 | 2 | 52 |
| Aug 2018 | 37 | 8 | 3 | 48 |
| Jul 2018 | 21 | 1 | 2 | 24 |
| Jun 2018 | 10 | 10 | 7 | 27 |
| May 2018 | 7 | 5 | 2 | 14 |
